Cloud Collaboration
for the Medical
Device Industry
Accelerate compliance, secure your IP,
and launch faster
and launch faster
Design Compliant Medical Electronics Faster with Secure Cloud-Based Collaboration
Medical Device Challenges
 How Altium 365 Helps
How Altium 365 Helps
Time-to-market pressure limits innovation and project scope.
- Accelerate development while maintaining quality and compliance.
Maintaining documentation for FDA, EMA, IEC, and ISO compliance consumes engineering time.
- Automatically document and track design changes to aid regulatory compliance.
Teams working in silos suffer coordination delays.
- Unite all stakeholders on a single secure platform.
Component lifecycle issues threaten long-term device viability.
- Access real-time supply chain data to ensure long-life component selection.
Sensitive IP is at risk when collaborating across distributed teams.
- Protect medical electronics designs with enterprise security and role-based access controls.
Reduce Risk, Speed Development,
Ensure Compliance
Ensure Compliance
Streamline Compliance & Traceability
Meet FDA, EMA, IEC, and ISO regulatory requirements with integrated data management, requirements management, and version control. Every design decision is tracked and traceable, creating audit-ready records that accelerate regulatory submissions and demonstrate compliance.
Protect Your Intellectual Property
Keep sensitive medical device designs secure from unauthorized access. Role-based access controls and end-to-end encryption allow distributed teams to collaborate on medical electronics development while protecting their valuable IP.
Manage Medical Electronics Lifecycle
Monitor component lifecycles and ensure regulatory compliance throughout a medical device’s service life. Real-time supply chain insights help you stay competitive and make longevity-focused design decisions down to the level of individual electronic components.
Reduce Time-to-Market for Medical Devices
Multi-disciplinary collaboration and process automation accelerate development. Real-time collaboration and integrated workflows help you deliver innovative medical electronics faster without compromising quality or risking costly rework.
Collaborate Throughout the
Medical Device Engineering
Process
Collaborate Throughout the
Medical Device Engineering
Process
Engineering Manager
Lead cross-functional teams efficiently and implement workflows that align with regulatory compliance at every stage. Oversee design milestones, validate processes, and coordinate seamlessly with medical device manufacturers from a centralized platform that supports FDA, EMA, IEC, and ISO compliance.
Learn More
Efficient
Cross-Functional
Team Collaboration
Centralized
Compliance
Management
System Architect
Link design requirements directly to regulatory standards. Track all design changes through secure version control, synchronize ECAD and MCAD data, and integrate seamlessly with cloud PLM systems.
Learn More
Enhanced Traceability &
Collaboration
Simplified
Compliance
Electrical Engineer
Design electronic systems and medical PCBs with a direct link between cloud data management and the industry’s best PCB design software. Manage component libraries, collaborate securely, and track design changes and reviews automatically.
Learn More
Streamlined
Design Process
Improved
Design Accuracy
Procurement
Ensure that design teams use approved components from approved vendors while proactively monitoring for end-of-life, availability, or pricing issues before they become a problem. Leverage detailed component data from top industry sources, seamlessly integrated into a dedicated BOM management tool.
Learn More
Reduce
Supply Chain Risk
Manage
BOMs with Ease
IT Security Manager
Protect intellectual property and sensitive design data throughout the development lifecycle. Leverage enterprise security protocols with granular permission controls, end-to-end encryption, and secure cloud storage for safe collaboration across distributed teams.
Learn More

Secure
Collaboration
Granular
Access Control
Key Capabilities
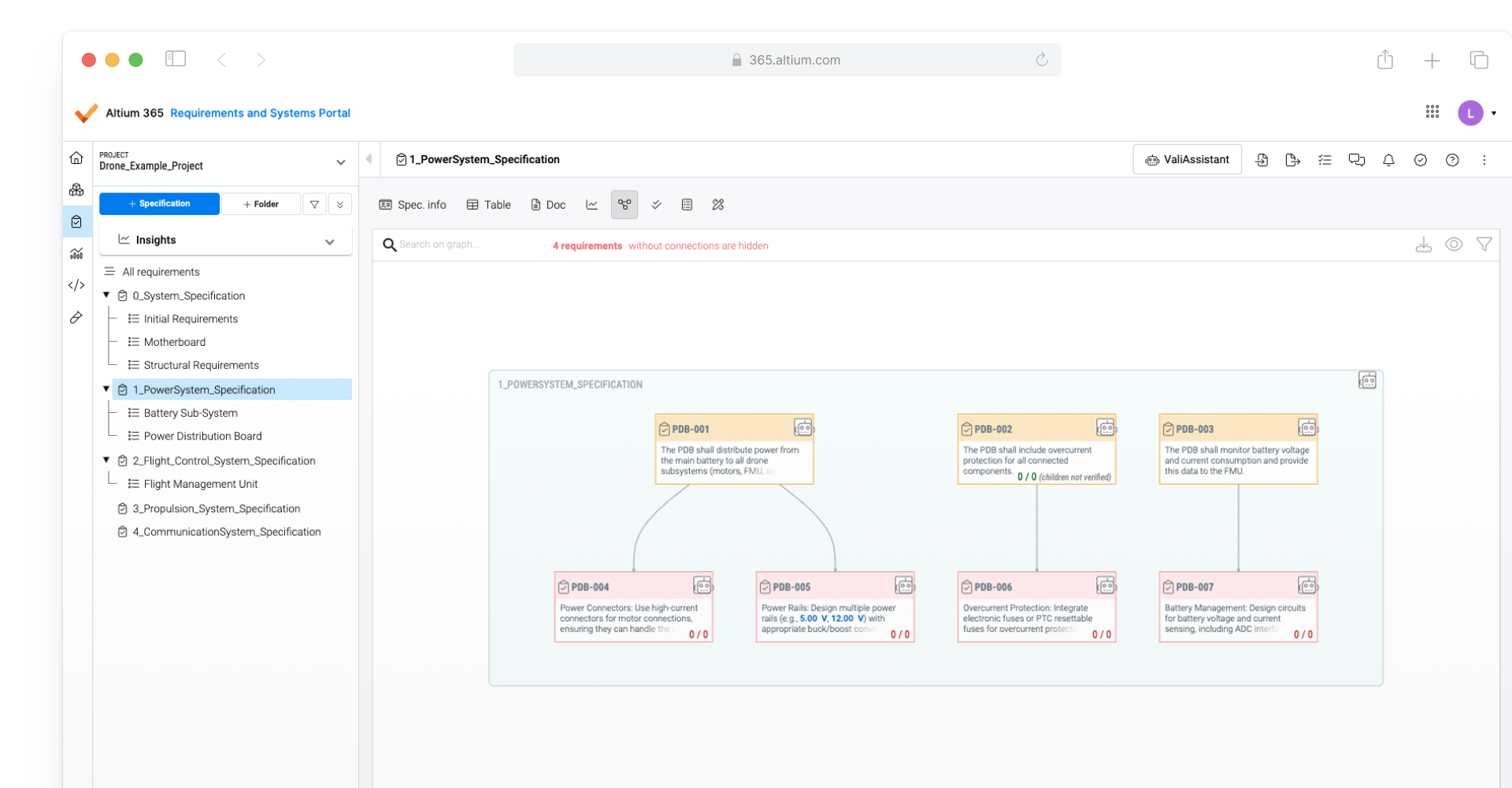
Link design data to regulatory requirements
Once medical device compliance regulations are translated into design requirements, Altium 365 Requirements & Systems can link these directly to design data. These compliance-related design requirements can be instantly verified and tracked throughout the product development lifecycle. This helps simplify proof of compliance with regulations like FDA 21 CFR Part 820, EMA, EU MDR, IEC 60601-1, 93/42/EEC, and ISO 13485, RoHS, and REACH.
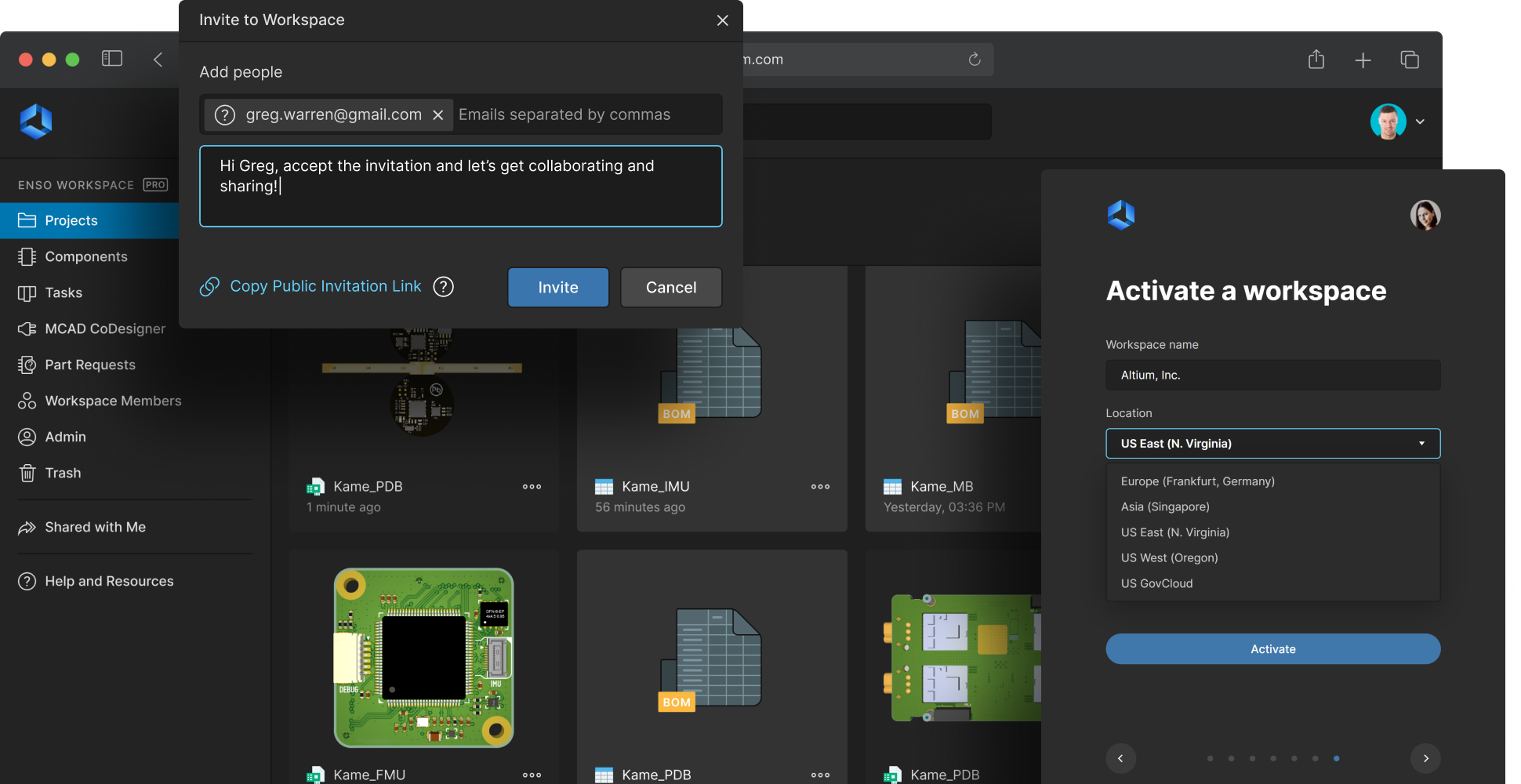
Secure medical electronics design data
Protect your intellectual property and design data. Altium 365 provides a secure, centralized repository for all design files, changes, and component information. End-to-end encryption and robust access controls ensure that only authorized users can access or modify your data. Teams collaborate seamlessly and confidently, knowing medical device engineering IP is protected against unauthorized access and security breaches.
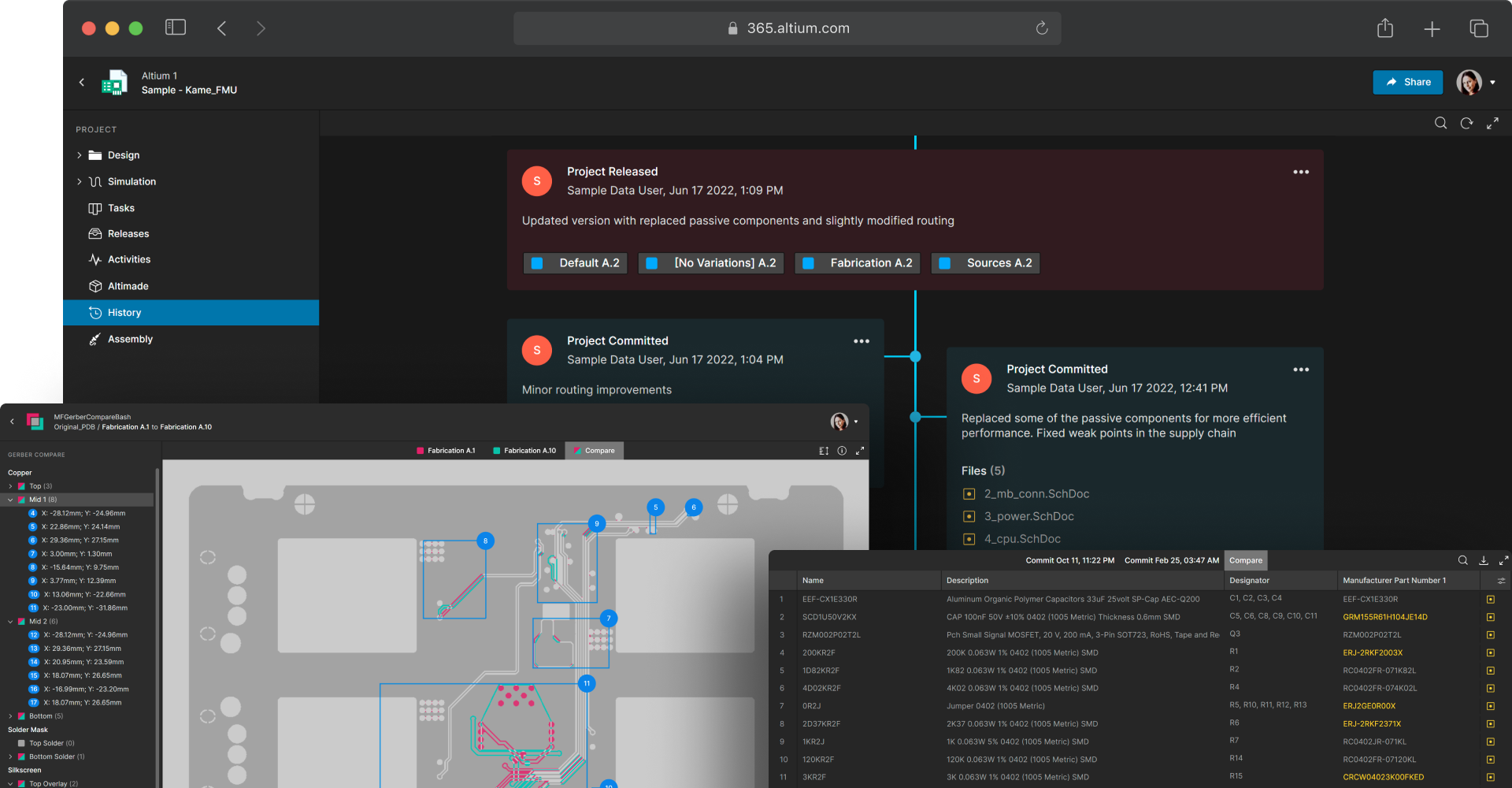
Maintain an auditable record of design changes
Medical device development requires traceability and transparency for every design modification. Each change must be tracked and documented to support regulatory compliance. Altium 365 version control and ECAD data management provide centralized data storage with hardware version control based on Git. You can easily compare schematic, PCB, Gerber, and BOM revisions for a complete record of what changes were made and by whom.
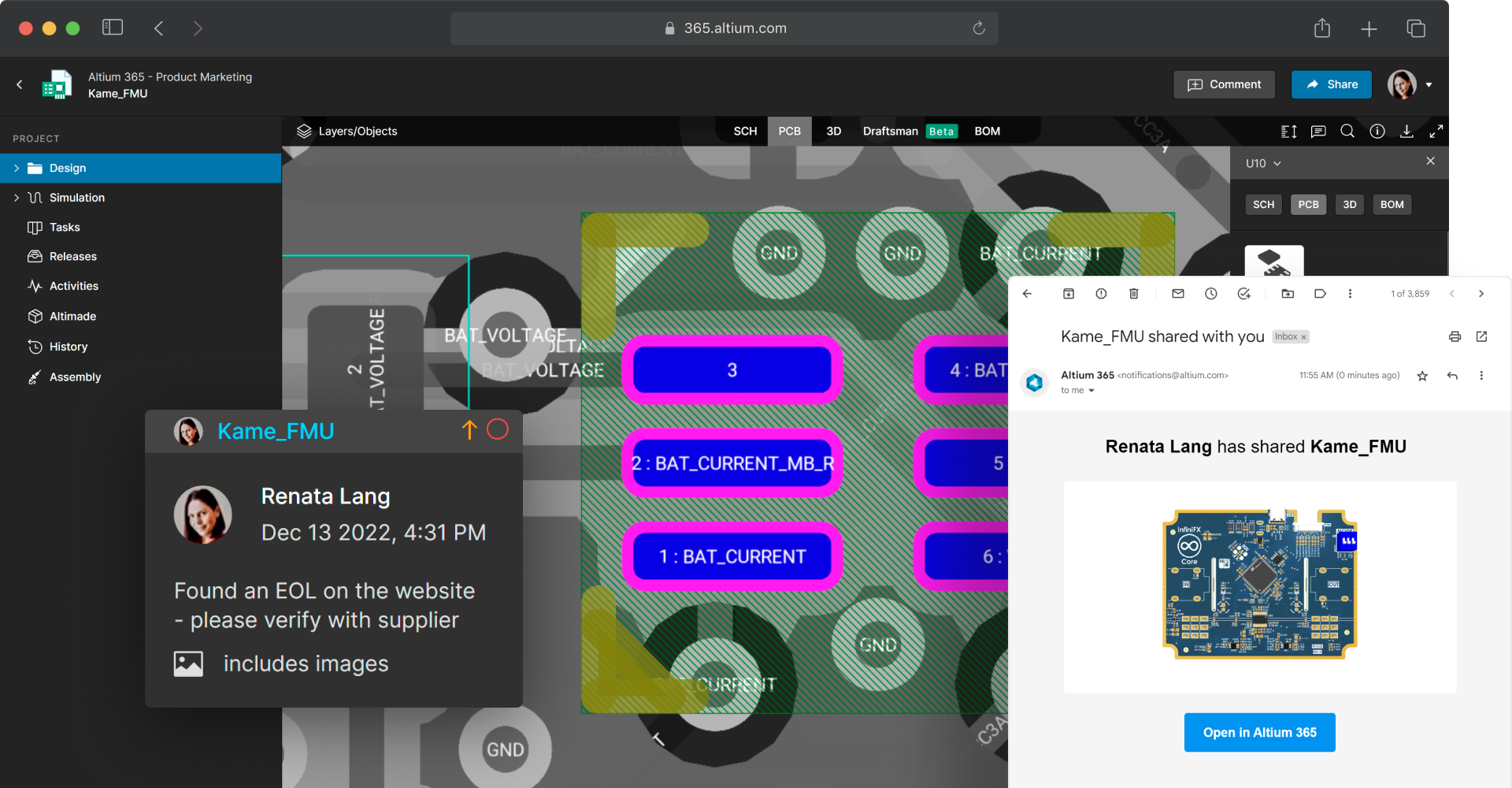
Coordinate design reviews with version-specific feedback
Invite stakeholders and track their feedback and approval form a single screen. Design reviews allow medical device designers and other expert stakeholders to review design documents in their browsers. Reviewers can leave contextual comments which are pinned to design assets, and tasks can be assigned to team members as design changes are identified. Once finalized, all review information is available in one place, and fields are non-editable but available for viewing for context and audit preparation.
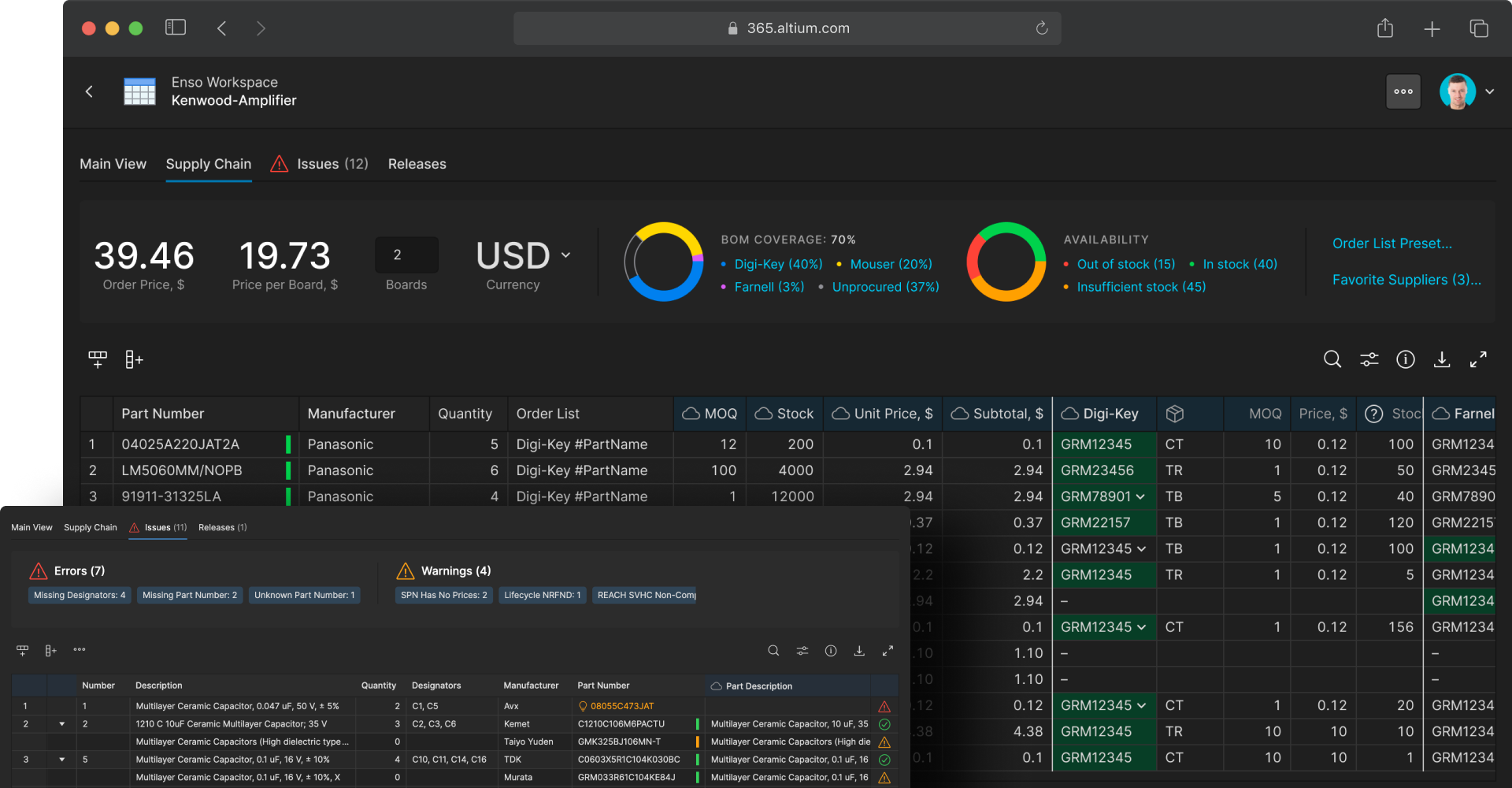
Monitor part prices and lifecycles with real-time BOM tracking
Many medical device electronics have long product lifecycles with near-perpetual uptime requirements, making component selection and lifecycle management critical. Altium 365 BOM Portal provides integrated supply chain management tools that allow electrical engineers to monitor component availability and pricing, plan procurement from multiple approved sources, and identify alternate parts when end-of-life becomes an issue. Real-time access to supply chain data and component lifecycle information helps teams make informed decisions about part selection, maintain product viability, and prevent supply chain disruptions.
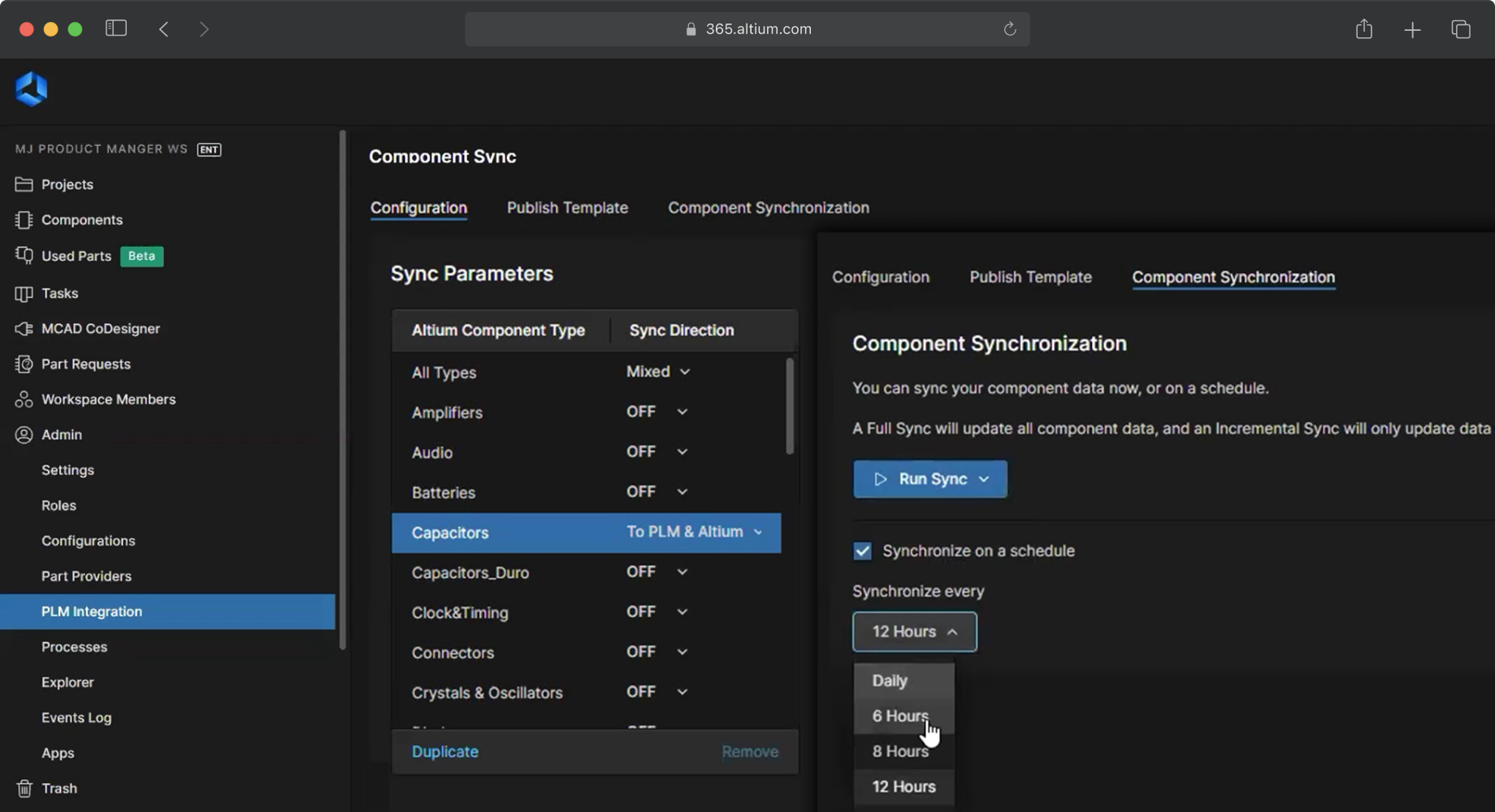
Unite electrical and mechanical design teams
Synchronize data between ECAD and MCAD systems to align mechanical and electronic design. Medical device development requires a high degree of collaboration among multidisciplinary teams, especially when the products are very small with tight tolerances. The MCAD CoDesigner application enables synchronized workflows with real-time updates and no loss of data accuracy between CAD packages. MCAD CoDesigner reduces iteration time and ensures that cross-functional requirements are met when the product goes to manufacturing.
Resources

Blog
Securing Medical Electronics IP in Collaborative Projects

Blog
Design Review Documentation Strategies for FDA-Auditable Medical Electronics

Blog
Understanding MDR: Impacts on Medical Device Development
Frequently Asked Questions
Altium 365 enables engineering teams to implement workflows that focus on compliance with FDA, EMA, IEC, and ISO standards. This is accomplished by automating certain portions of design documentation, thanks to a built-in version control system. Altium 365 Requirements & Systems Portal also provides requirements traceability and allows engineering management to specify design requirements in terms of regulatory requirements. These aspects of documentation are part of the comprehensive audit trails that are essential for regulatory compliance.
All design change histories are tracked through integrated version control. Whenever a design is modified and entered into version control, designers can include documentation outlining specific changes to the design. This information is a critical portion of a complete, auditable record that tracks who made changes, when they were made, and why—essential for medical electronics manufacturing compliance.
Altium 365 MCAD CoDesigner allows mechanical engineers to access electrical designs and make changes to the mechanical layout using popular MCAD applications, such as SolidWorks. The mechanical changes can then be pushed back to the electrical designer. This push-pull data exchange process happens inside the respective design applications which accelerates the design process and eliminates data errors caused by file exports that have historically been required for electrical and mechanical design collaboration.
Yes, Altium 365 works with Altium Designer, OrCAD®, KiCad®, Eagle®, and PADS® files. Many companies have design files in various ECAD formats they have used over the years. Altium 365 enables collaboration on design reviews and BOM management while tracking everything in version control, ensuring all ECAD information is in one place for easier tracking and audit preparation. Learn more about efficient collaboration in a multi-cad environment.

Ready to streamline
medical electronics
development?
Get secure cloud collaboration purpose-built
for medical electronics engineering teams.
- Streamline compliance and traceability
- Protect your intellectual property
- Manage medical electronics lifecycle
- Reduce time-to-market for medical devices
Complete this form, and our electronics development experts will contact you soon.


